Hypoxia-induced chemoresistance
These projects aim at better understanding the cell responses to hypoxia, including the mechanisms underlying the activation of the major transcription factor activated by hypoxia, HIF-1 (hypoxia-inducible factor-1). A major research axis of our group is the study of the effects of hypoxia on cancer cell survival. These responses allow cancer cells to survive the hypoxic conditions developing in the tumor microenvironment but also to become resistant to chemotherapy. For example, we have shown that hypoxia protects cancer cells from the apoptosis induced by etoposide or taxol. In addition, hypoxia also inhibits p53 activation, thus preventing apoptosis to be initiated. The role of several proteins of the Bcl-2 family has also been investigated. Moreover, we have also shown that etoposide induces autophagy that participates to cell death under normoxia but not under hypoxia, probably through a different regulation of BNIP3 expression. The involvement of autophagy as well as of the Unfolded Protein Response has also been demonstrated for the protection against taxol-induced cell death in breast cancer cells exposed to hypoxia. Finally, several miRNA modulating cancer cell chemosensitivity have been unraveled.
Selected publications
- Rebucci M, Sermeus A, Leonard E, Delaive E, Dieu M, Fransolet M, Arnould T & Michiels C. 2015, ‘miRNA-196b inhibits cell proliferation and induces apoptosis in HepG2 cells by targeting IGF2BP1’ Mol Cancer, vol 8, pp. 79-96.
- Notte A, Rebucci M, Fransolet M, Roegiers E, Genin M, Tellier C, Watillon K, Fattaccioli A, Arnould T & Michiels C. 2015, ‘Taxol-induced unfolded protein response activation in breast cancer cells exposed to hypoxia: ATF4 activation regulates autophagy and inhibits apoptosis’ Int J Biochem Cell Biol, vol 62, pp; 1-14.
- Sermeus, A, Rebucci, M, Fransolet, M, Flamant, L, Desmet, D, Delaive, E, Arnould, T & Michiels, C 2013, 'Differential effect of hypoxia on etoposide-induced DNA damage response and p53 regulation in different cell types' Journal of Cellular Physiology, vol 228, pp. 2365-2376.
- Notte, A, Ninane, N, Arnould, T & Michiels, C 2013, 'Hypoxia counteracts taxol-induced apoptosis in MDA-MB-231 breast cancer cells: Role of autophagy and JNK activation' Cell Death and Disease, vol 4, no. 5, pp. e638.
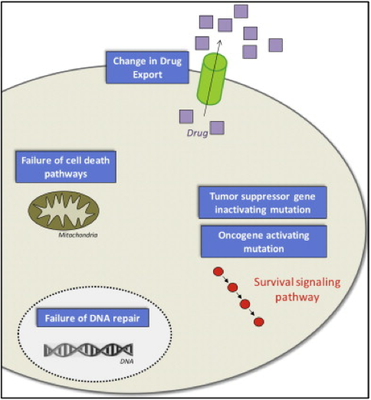
- Rebucci, M & Michiels, C 2013, 'Molecular aspects of cancer cell resistance to chemotherapy' Biochemical Pharmacology, vol 85, no. 9, pp. 1219-1226.
- Sermeus, A, Genin, M, Maincent, A, Fransolet, M, Notte, A, Leclere, L, Riquier, H, Arnould, T & Michiels, C 2012, 'Hypoxia-induced modulation of apoptosis and BCL-2 family proteins in different cancer cell types' PLoS ONE, vol 7, no. 11, pp. e47519.
- Notte, A, Leclère, L & Michiels, C 2011, 'Autophagy as a mediator of chemotherapy-induced cell death in cancer' Biochemical Pharmacology, vol 82, pp. 427-434.
- Sermeus, A & Michiels, C 2011, 'Reciprocal influence of the p53 and the hypoxic pathways' Cell Death and Disease, vol 26, pp. e164.
- Cosse, J-P, Rommelaere, G, Ninane, N, Arnould, T & Michiels, C 2010, 'BNIP3 protects HepG2 cells against etoposide-induced cell death under hypoxia by an autophagy-independent pathway' Biochemical Pharmacology, vol 80, no. 8, pp. 1160-1169.